Our research aims to discover, develop and understand new transition-metal-catalyzed organic transformations of broad interest to organic synthesis. We are particularly interested in the design of efficient catalytic systems that operate through uncommon modes of activation and which will result in a rapid increase in molecular complexity.
The following topics will be investigated in my group:
Transition-metal-free C-C/C-B bond formation reactions
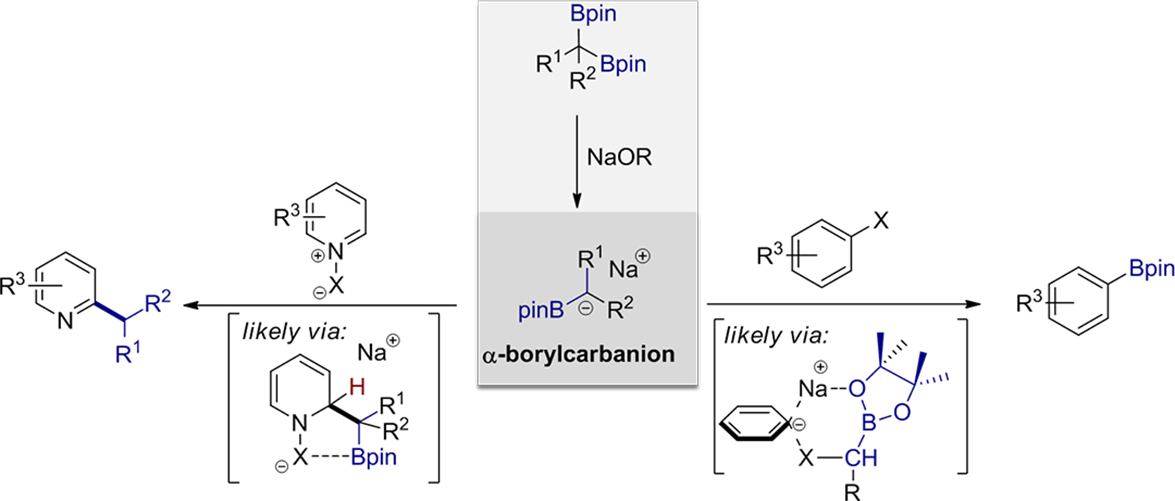
We have observed that α-borylcarbanion, generated in situ from the reaction of 1,1-diborylalkane with an alkoxide base, could facilitate an unprecedented base-promoted deborylative alkylation of N-heteroarenes. The reaction proceeds efficiently for a wide range of N-heteroarenes and 1,1-diborylalkanes with excellent regioselectivity. The reaction also can be applied for the direct introduction of a methyl group to 9-O-methylquinine N-oxide, thus it can serve as a powerful method for late-stage functionalization. Moreover, a new transition-metal-free borylation of aryl and vinyl halides using 1,1-diborylalkanes as boron sources is also developed. In this transformation one of the boron groups from 1,1-diborylalkanes is selectively transferred to aryl and vinyl halides in the presence of sodium tert-butoxide as the only activator to form organoboronate esters. Under the developed borylation conditions, a broad range of organohalides are borylated with excellent chemoselectivity and functional group compatibility, thus offering a rare example of a transition-metal-free borylation protocol.
Transition-metal-catalyzed chemo-, regio-, and stereoselective C-C bond formation reactions
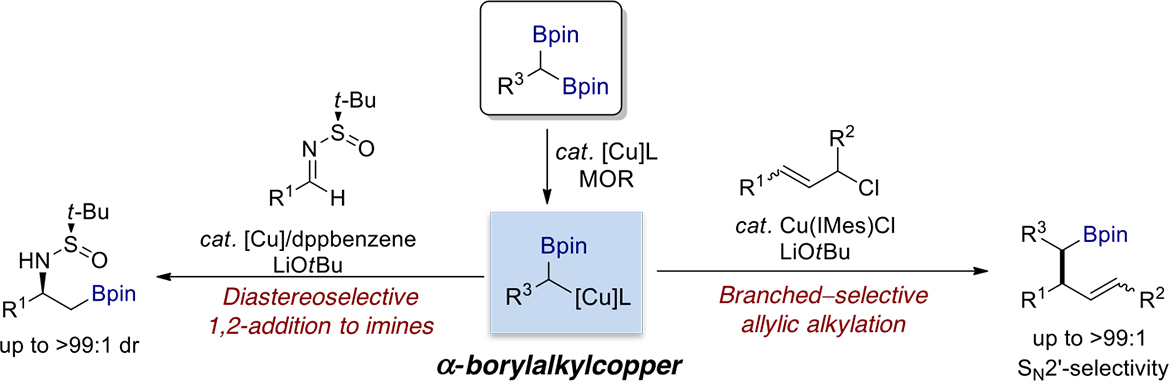
We have observed that the chemoselective transmetalation of 1,1-diborylalkanes with copper catalyst can be achieved in the presence of alkoxide base. The generated a-borylalkylcopper species underwent a highly chemo- and diastereoselective alkylation of N–tert-butanesulfinyl aldimines with diborylmethane to form chiral β-aminoboronates, which are valuable synthetic building blocks in organic synthesis. The developed copper-catalytic system was also extended to a copper-catalyzed SN2′-selective allylic substitution reaction using readily accessible allylic chlorides and 1,1-diborylalkanes, a reaction which proceeds with chemoselective C−B bond activation of the 1,1-diborylalkanes. This method provided an useful approach to afford branched alkylboronate esters.
Design and synthesis of new multi-organometallics and their applications to the enantioselective reactions